Li Bai
February 5, 2024
I’ve always thought that the person who best captured the spirit of traveling in China is Bill Porter (Chinese name ‘Red Pine’), who does translations of Chinese poems. His notable books include South of the Yangtze and Finding Them Gone, the latter getting its title from the frequent lament of Chinese poets who arrive at a long-sought destination to meet a colleague or friend, only to be disappointed.
One frequent subject of Porter’s books is Li Bai (or Li Pai or Li Po in Western translations), a famous poet from the Tang Dynasty whose poems are memorized by all Chinese school children. One particularly interesting Western tribute is Ezra Pound’s beautiful & touching The River-Merchant’s Wife: A Letter… a poem ‘after Li Po.’ Pound revolutionized modern poetry, and published a well-known collection Cathay dedicated to such translations….. even though Pound did not speak any Chinese! More recently, Li Bai was the subject of a 2019 biography from the National-Book-Award winning author Ha Jin.
There are two things likely to be found in any Li Bai poem: his love of drinking wine & spirits, and the moon. Not surprising, then, the legend of his passing links these topics: supposedly, Li was out in a boat on the Yangtze River, and saw a reflection of the moon on the water – and in his inebriated effort to capture it, fell overboard and drowned. The site of that drowning is memorialized at Caishiji Park in Ma’anshan, a city about 35 kilometers from Nanjing, and it’s long been on my “must-see” list – yet another China site visit disrupted by covid!

Luckily, an HNC law student in one of my ERE classes, Ms. Chen Junjie, was kind enough to take me there this semester, acting as a guide and helping me locate Qian Shaowu's well-known statue of Li Bai, showing him in flight as an immortal. We also headed to another site a further 20 kilometers away, the tomb where at least some of his remains are reportedly buried. That location had an aroma more like a distillery, however, as those paying homage – including Bill Porter and his colleagues – typically douse the tomb with liquid offerings.

Hamburg
September 30, 2023
I came back to China via Europe, and stopped in to see my good friends Janosch & Jana – both Ph.D. environmental economists whom I’ve known for many years, and who have welcomed me on various vacation stays in Tuscany, Southern France, etc. They live in Buchholz, a town just south of Hamburg – and this was my chance to visit a city that I’m afraid I knew very little about…. other than that the Beatles had spent time and played there before becoming world famous.
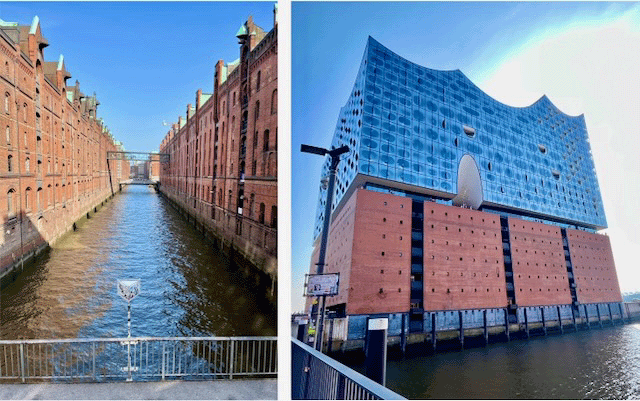
Hamburg sits about a hundred kilometers from the North Sea on the Elbe River, and I had always thought of it as an in-land city – never realizing the importance of its port (in both historical and present-day terms). I had also never realized how lively and beautiful it is – with parks and old-time port buildings, modern architecture like the Elbphilharmonie, and a vibrant nightlife in the red-light Reeperbahn district (where the Beatles played in joints typical of a port city).

Following up on my recent London & Liverpool visit, we headed to Beatles-Platz one warm evening, to see the sights. It’s a bit difficult to capture and appreciate the ‘see-through’ Beatles statues in the photo above – as well as the fact that the black circular setting is shaped like a giant vinyl record. But the photo does give some indication that the Reeperbahn remains a lively place!
Other site visits included the city-center Binnenalster lake (with its notable fountain, seen in the initial photo above); the City Hall ‘Rathaus’; the former city wall area, now transformed into a scenic city park; the UN World Heritage Site historic port area; and the spire remains of the old St. Nicholas church – now a memorial of the city’s bombing in World War II.
I was visiting Hamburg on my way to China, getting ready to teach my environmental course (over-subscribed, with 39 students signed up!) – and took along a very recent book, Dan Egan’s The Devil’s Element, about the worrisome pollutant phosphorus. Phosphorus is one of two pollutants (the other being nitrogen) that cause massive algal blooms in lakes and offshore areas around the world, and the element has become a major pollution concern. What I did not realize, however, was that Hamburg would play a major role in the book!
An early chapter in this fascinating work describes the discovery of phosphorus by the alchemist Hennig Brandt in the city in 1669. Brandt was a failed glass-maker who became a self-proclaimed Doctor (of both medicine & philosophy) – despite having no formal education. He isolated phosphorus with a recipe that involved boiling vats of urine, but was never able to take the ultimate desired step – to turn it into gold. It is extremely flammable, however, and can spontaneously ignite if exposed to air -- burning both equipment and any nearby flesh, making its production a very risky endeavor!

Such characteristics led to the story of that bombed-out church memorial. The Allies used phosphorus in their bombs, which were specifically designed to maximize the incendiary effects of such weapons. The death toll from Operation Gomorrah in Hamburg is not precise, since in some cases no bodies remained; it burned so hot that physicians weighed ashes to estimate the fatality rate (estimated at about 38,000 persons).
Egan’s book ends with a company building a state-of-the-art phosphorus recovery plant in the city, on the west bank of the Elbe River, to tackle this very necessary mineral – but also current environmental scourge. The devil’s element indeed!
Joseph Priestley House
August 25, 2023
I begin my energy course at HNC every year with a discussion about combustion, since it makes up about 82% of primary energy (still!). We burn coal, oil, natural gas, trash, wood, agricultural waste, and much, much more. Combustion is a chemical reaction (rapid oxidation), and that in turn leads us to review the work of Joseph Priestley, who discovered the gas oxygen, and Antoine Lavoisier, who gave it the name “oxygen” and recognized its role in the combustion process.

Priestley was a Unitarian preacher in the U.K. who was nonetheless a world-class chemist. His “non-conformist” religious and liberal views got him into trouble, and his house was burned down by a mob. He fled to Philadelphia in the U.S. in 1794, and ultimately settled in Northumberland, Pennsylvania, a town on the Susquehanna River – at the time a five-day trip from the city.
It was only a three-hour drive for me, however – and so I made the trip to Joseph Priestley’s House to see the museum and laboratory during an August sojourn to a family reunion in the western part of the state.
Priestley had met Lavoisier in Paris in 1774, and described his experiments – which Lavoisier subsequently repeated. Prior to that time, chemists had believed in “phlogiston”, a substance present in combustible materials and released into the air during burning. Lavoisier’s insights overturned that view and revolutionized chemistry, although Priestley remained a phlogiston believer until the end of his life. [He had labeled oxygen “dephlogisticated air,” so we’re lucky that Lavoisier’s chemical ideas ultimately prevailed!] Lavoisier revolutionized chemistry, but he ultimately lost his head to the guillotine during the French Revolution.
Their stories are well-told in a couple of books that I recommend to my students, shown below. Despite his combustion reticence, Priestley was a brilliant chemist, and he published more than thirty scientific papers during his stay in Northumberland – including discovery of the gas carbon monoxide (with another unfortunate label: “heavy inflammable air”). The American Chemical Society has noted that his library (with some 1600 volumes) and chemical laboratory “were probably the best in the country at that time,” and his domicile has now been designated a National Historic Chemical Landmark.



